Influence of Chemical Properties of Soil on the Corrosion Morphology of Carbon Steel Pipes
ABSTRACT:
Corrosive soils are responsible for the deterioration of buried underground utilities such as buried steel pipes. Frequent pipe failures are reported due to corrosive soil globally. Although soil’s corrosion phenomenon has been understood and identified long time ago, pipe failures due to corrosive soil are uncontrollable and unavoidable despite the use of protective coatings and techniques such as cathodic protection. Therefore, it is essential to review the causes of soil’s corrosivity for the protection of steel pipes. This chapter demonstrates the influence of varying moisture and chloride contents of soils on the corrosion of coated and uncoated steel pipes. Carbon steel specimens (coated and uncoated) were buried in soils of 20, 40, 60, and 80 wt.% moisture content, respectively, while the chloride concentration introduced in soil was 0, 5, and 10 wt.%, respectively. Through the analysis of experiments, it is revealed that the corrosion rate of pipes buried in soil increases with increase in moisture content up to critical moisture and chloride values. The influence of soil’s moisture and chloride on the corrosion products formed on steel pipes was investigated and comprehensively explained in this chapter. Authors believe that the knowledge presented in this chapter can be applied to other structures or utilities buried in corrosive soils.1. INTRODUCTION:
The influence of soil’s chemical properties is reported as the root cause of failures of buried pipes. The chemical constituents of soil react with the surface of unprotected buried pipes, which in turn results in the corrosion of pipes. However, there is still no complete preventive solution to the corrosion caused by the chemical constituents of soil even in the presence of advanced corrosion protection techniques.As per above referred studies, soil’s constituents cause corrosion of buried pipes; these include moisture contents, pH, temperature, soil resistivity, soil type, soil particle size, permeability, differential aeration, and sulphate-reducing bacteria (see references above). Researchers have adopted various approaches based on field testing (all above references) and experiments to investigate these factors. Soil has been reported as the main stimulants causing failure of buried metallic pipes in Fig 3
 |
| Fig. 1:: Worldwide causes of corrosion of metallic buried pipes (redrawn, originally by [3]). |
The other notable chemical constitute of the soil, i.e., chloride, well known for its corrosion-causing capability particularly to reinforce concrete structures can be responsible to the failure of steel pipes. However, from the comprehensive reviews, it can be found out that the research related to the effect of chlorides present in soil and the corresponding corrosion of carbon steel pipes is limited. Considering this gap, current research is conducted in which varying quantity of moisture and chlorides contents of the soil are taken into consideration for finding their effect and a coupled threshold value which would be useful to determine the service life of buried pipes.
2. EXPERIMENTAL METHODOLOGY:
The micro-structure of corroded carbon steel samples exposed to different moisture and chloride conditions of soils was investigated. Authors conducted an experimental study on corrosion behavior of carbon steel and zinc-electroplated and copper-electroplated carbon steels. Carbon steel specimens were exposed to 20, 40, 60, and 80 wt.% moisture, respectively, and the chloride concentration was kept at 0, 5, and 10 wt.%. First, the coupled effect of moisture and chloride which induced the maximum corrosion rate was evaluated. Then, zinc-electroplated and copper-electroplated steel specimens were exposed under similar aggressive coupled moisture and chloride condition. The details of the soils and specimen preparations and the chemical composition of the steel and soil used can be found elsewhere.Experiments were performed under laboratory-controlled temperature of 27 ± 1°C. The set-up for the electrochemical measurements consisted of three electrode cells. The schematic diagram of the experimental test set-up is shown in Figure 2. In this figure, the specimen is represented by rectangular shape; was a carbon steel without coating, and with zinc- and copper-electroplated coatings, respectively; and was used as working electrode. In addition, two counter electrodes made of graphite and joined through electrical connector and copper/copper sulphate (Cu/CuSO4) solution as reference electrode (RE) were used, respectively, for corrosion measurement of each specimen used in the current research. The surface area ratio between working electrode/counter electrode was kept at 0.909. Electrochemical measurements, i.e., electrochemical impedance spectroscopy (EIS), were performed using Autolab PGSTAT302N potentiostat/galvanostat. Before performing electrochemical measurement, metallic samples were cleaned as per ASTM G1-03. Then each specimen was buried into 1000 g of soil containing controlled moisture contents, chloride concentrations, and combination of both as discussed earlier. The open circuit potential of working electrode was observed for 3600 s. More details of the EIS and potentiodynamic polarization procedures executed in the current research can be found in author’s recent publication. The EIS data was fit by using Nova 1.1.1 software for corrosion analysis. After knowing the corrosion rates of specimens, deep micro-structure analysis of the corrosion morphology of all the specimens was executed using scanning electron microscopy (SEM). The corrosion results obtained and the related discussion are presented in the following section. More details about the preparation of specimens and soils can be found elsewhere.
 |
| Fig. 2:: Set-up for electrochemical measurement (modified after Shoaib et al. [15]). |
3. RESULTS AND DISCUSSIONS:
3.1 Electro-Chemical study on SS400 Carbon Steel:
The electrochemical study is carried out to investigate the corrosion behavior of metals. It has been examined the influence of moisture and chloride on the corrosion behavior of SS400 carbon steel in the soil environment.Electro-Chemical results showed that the corrosion rate of SS400 carbon steel sample increased with increase in moisture content up to 60 wt.% and decreased after this value. Moreover, with the addition of chloride, corrosion rate increased appreciably. The maximum corrosion rate was noticed for carbon steel exposed to soil containing 60 wt.% moisture and 5 wt.% moisture. Theoretically, carbon steel specimen buried in 60 wt.% moisture and 10 wt.% chloride should have more corrosion rate because of exposure of higher chloride content. Probably, the possible reason could be a non-homogeneous nature of the soil, or there is a possibility that organic contents might have caused the increase in soil’s resistivity and hence the decrease in corrosion rate even in the presence of higher chlorides.
The corrosion rates of carbon steel samples under different exposure conditions are shown in Figure 3. The detailed discussion on the corrosion behavior of various specimens can be found elsewhere. After Electro-chemical measurements of various steel samples, they were examined using laser microscope, scanning electron microscope (SEM), and energy-dispersive X-ray spectroscopy (EDS) to investigate the influence of chemicals in soils on the micro-structure of buried steel samples. The discussion is presented in the following section.
 |
| Fig. 3:: Corrosion rates of SS400 steel samples under different moisture and chloride conditions (modified after Shoaib et al). |
3.2 Microscopic Observation:
First, the coupled effects of varying moisture and chloride on corrosion were measured; then their subsequent impact on the micro-structure of specimens was investigated using Olympus laser microscope. Corrosion patterns on specimens after exposure to various corrosive soils are shown in Figures 4, 5, 6; from these figures red rust can be seen on all samples. Interestingly, there was a further addition of red rust with the increase of chloride contents indicating the presence of iron oxides on the surface. Moreover, optical images also confirmed that the addition of chloride promoted corrosion progress. With further addition of chloride, more red rust was observed. |
| Fig. 4:: Corrosion morphology of carbon steels tested in different soil moisture contents (a = 20 wt.% MC; b = 40 wt.% MC; c = 60 wt.% MC; d = 80 wt.% MC). |
 |
| Fig. 5:: Corrosion morphology of carbon steels tested in soil with 5 wt.% chloride a = 20 wt.% MC; b = 40 wt.% MC; c = 60 wt.% MC; d = 80 wt.% MC). |
 |
| Fig. 6:: Corrosion morphology of carbon steels tested in soil with 10 wt.% chloride (a = 20 wt.% MC; b = 40 wt.% MC; c = 60 wt.% MC; d = 80 wt.% MC). |
 |
| Fig. 7:: Corrosion morphology of (a) copper-electroplated and (b) zinc-electroplated steels tested in soil with 60 wt.% moisture and 5 wt.% chloride. |
3.3 Corrosion Product Morphology
SEM analyses were performed after the samples were corroded to various soil conditions. Figure 8 shows the SEM micrographs of low and high magnifications of carbon steel. From this figure, a porous and honeycomb-like structure appeared, which is also reported by earlier researchers. Figure 9 shows EDS spectra of elements present in corrosion product of carbon steel, while the elements in the corrosion layer of carbon steel are shown in Table 1. The presence of sodium (Na) on a metallic surface, indicating that cation in soil penetrated through corrosion product layer and reached the sample’s surface. As a result of this penetration, the corrosion process is accelerated. However, it has been reported in literature that the initial corrosion product formed on carbon steel is α-FeOOH which provides a shield to substrate metal against corrosion. |
| Fig. 8:: SEM images of carbon steel buried in soil of 60 wt.% moisture and 5 wt.% chloride. |
 |
| Fig. 9:: EDS spectra of carbon steel tested in soil with 60 wt.% moisture and 5 wt.% chloride. |
 |
| Table 1:: EDS analyses (wt.%) of carbon steel of selected spots in Figure 8. |
 |
| Fig. 10:: SEM images of zinc-coated steel tested in soil with 60 wt.% moisture and 5 wt.% chloride. |
 |
| Fig. 11:: EDS spectra of zinc-coated steel tested in soil with 60 wt.% moisture and 5 wt.% chloride. |
 |
| Table 2:: EDS analyses (wt.%) of zinc-coated steel for selected spots in Figure 9 |
 |
| Fig. 12:: SEM images of copper-coated steel tested in soil with 60 wt.% moisture and 5 wt.% chloride. |
 |
| Fig. 13:: EDS spectra of copper-coated steel tested in soil with 60 wt.% moisture and 5 wt.% chloride. |
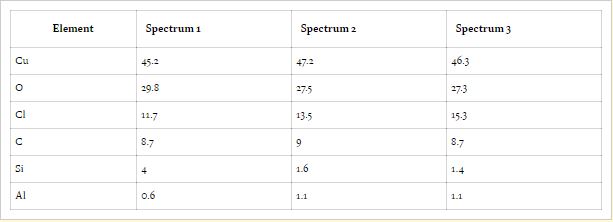 |
| Table 3:: EDS analyses (wt.%) of copper-coated steel for selected spots in Figure 12. |
The presence of Cl element in the corrosion layer of carbon, zinc-coated, and copper-coated steels was obviously due to the addition of NaCl. The Cl anion is classified as aggressive because it directly contributes in electrochemical reaction causing corrosion. Cl anion present in coated samples reveals that it has a strong tendency to promote corrosion rate even if the metallic surface is coated with zinc or copper.
3.4 Energy-dispersive X-ray spectroscopy (EDS) mapping
Cross-sectional EDS map of specimens buried in soil containing 80 wt.% moisture is shown in Figure 14a and b. In Figure 14a, O-K and Fe-K element maps show that oxygen and iron are uniformly distributed. The co-existence of O and Fe elements demonstrates that oxides of iron are present in corrosion products. Generally, α-FeOOH and γ-FeOOH are observed as corrosion products of steel buried in the soil environment. A C-K element from epoxy resin has a non-uniform distribution. At site 2 (Figure 14b), there is an excessive concentration of C-K due to the epoxy resin, and there is less concentration of O-K and O-K at this site. |
| Fig. 14:: (a) EDS map of carbon steel exposed to soil containing 80 wt.% moisture (site 1). (b) EDS map of carbon steel exposed to soil containing 80 wt.% moisture (site 2). |
Figure 15a and b demonstrates cross-sectional EDS map of carbon steel sample exposed to soil containing 60 wt.% moisture and 5 wt.% chloride at site 1. A pit can be observed on carbon steel sample. It confirms that exposure of carbon steel to soil containing chloride accelerates the corrosion; as a result pitting is observed. The layer of oxygen has a variable thickness, and C layer from epoxy has a non-uniform thickness due to which no chloride contents were observed. The possible reason is that any element having a concentration less than 1% cannot be mapped by EDS mapping technique because peaks of elements having less concentration are difficult to separate from the background. Figure 15b shows EDS mapping at site 2, where the pit is less wide than site 1.
Theoretically, carbon steel specimens exposed to 10 wt.% chloride should be more corroded than 5 wt.% chloride; however, experimentally this was not observed. The reason for this phenomenon is probably due to the fact that soil is a non-homogeneous, and its properties vary within the soil itself. There is a possibility that soil sample used for 60 and 5 wt.% chloride conditions might have considerable chloride already present in it. Furthermore, it is also possible that the soil sample used for 60 and 10 wt.% might have organic contents. The presence of organic contents in soil increases the soil resistivity and ultimately decreases the corrosion rate. It is concluded from the above discussion that the addition of chloride in soil accelerates the corrosion rate significantly and in a short time. The increase in exposure duration to chloride-contaminated soil can lead to pitting of carbon steel.
4. CONCLUSIONS:
In this chapter, a study related to the influence of soil’s varying moisture and chloride contents on the corrosion and subsequent micro-structure of coated and uncoated carbon steel pipes is presented. From the experimental findings of corrosion and then after carrying out micro-structural analysis, a threshold value for moisture and chloride contents is determined beyond which no further addition of chloride and moisture contents can cause corrosion and deterioration of micro-structure of carbon steel. The results presented in this paper have practical application for the protection of coated and uncoated carbon steel pipes in soils. This study can help owners of the steel pipes to decide which type of coating to be used for the protection of the carbon steel pipes in aggressive soil conditions such as those presented in this paper. The current research is further extended for longer exposures and evaluating the influence of corrosion on the mechanical properties of buried steel pipes.
Courtesy: Muhammad Waseem and Shahrukh Shoaib
SIX STEPS TO PROTECT RUSTING OF PIPES
Rust is the name for the orange-brown flakes of iron oxide that form on the surface of any metal containing iron that is exposed to air and water. It is a type of corrosion that can be highly destructive, as well as unsightly. In this article, we will share tips on how to prevent rust.
The rusting process begins when iron reacts with oxygen in the presence of water, saltwater, acids, or other harsh chemicals. As the iron oxide flakes off the metal surface, it exposes fresh iron molecules, which continue the reaction process. Eventually, large areas of rust form that may cause the entire metal structure to disintegrate.
A ferrous metal is one that contains iron and only iron can rust. Common ferrous metals include carbon steel, alloy steel, and stainless steel. Non-ferrous metals, such as aluminum and copper, contain little if any iron, and so cannot rust, though they can corrode.
1. Keep It Clean and Dry
Water is enemy number one when it comes to rust, because it’s the oxygen in water molecules that combines with iron to form iron oxide. That’s why metals left outdoors, such as cars, gates, or tanks, are more likely to rust. If the object is located in a humid indoors environment, such as a garage or basement, install a dehumidifier. Any type of mud or dirt adhered to the surface can hold water, so it’s important to keep metals clean.
2. Prevent Scratches
Scratches or cracks in the metal expose more metal and hold water, allowing it to remain in contact with the iron. This is why cold rolled steel is more corrosion resistant than hot rolled steel, because cold rolling creates a smoother surface without texture that can trap and hold water.
3. Apply A Protective Coating
Dipping metal objects, such as clocks, into a bluing solution of water, sodium hydroxide, and potassium nitrate, provides strong corrosion resistance. Commercially available rust prevention products in the form of aerosol sprays or cloth wipes also can protect metal objects, including tools, outdoor gear, vehicles, and large metal parts.
4. Use Stainless Steel
Stainless steel alloys contain iron, but it resists rust because it also contains a high percentage of chromium which is even more reactive than iron. The chromium in the alloy oxidizes quickly to form a protective layer of chromium oxide on the metal surface which prevents oxygen from reaching the underlying steel.
5. Use Galvanized Metal
Galvanization is a process used to preserve steel rust-free for many years. In the galvanizing process, a piece of steel is coated with liquid zinc. The zinc protects the steel in three different ways. First, the zinc coating acts as a barrier preventing oxygen and water from reaching the steel. Second, even if the coating is scratched off, the zinc continues to protect nearby areas of the metal through cathodic protection. And third, zinc is highly reactive to oxygen and quickly forms a protective coating of zinc oxide which prevents the iron from further oxidation.
6. Regular Maintenance
Because rust spreads quickly, it’s important to scrape it off as soon as it appears. Then, scrub with warm water and soap and apply a metal conditioner or other protective coating to prevent further oxidation. If necessary, apply a new coat of paint to the area.
4. CONCLUSIONS:
In this chapter, a study related to the influence of soil’s varying moisture and chloride contents on the corrosion and subsequent micro-structure of coated and uncoated carbon steel pipes is presented. From the experimental findings of corrosion and then after carrying out micro-structural analysis, a threshold value for moisture and chloride contents is determined beyond which no further addition of chloride and moisture contents can cause corrosion and deterioration of micro-structure of carbon steel. The results presented in this paper have practical application for the protection of coated and uncoated carbon steel pipes in soils. This study can help owners of the steel pipes to decide which type of coating to be used for the protection of the carbon steel pipes in aggressive soil conditions such as those presented in this paper. The current research is further extended for longer exposures and evaluating the influence of corrosion on the mechanical properties of buried steel pipes.Courtesy: Muhammad Waseem and Shahrukh Shoaib
SIX STEPS TO PROTECT RUSTING OF PIPES
Rust is the name for the orange-brown flakes of iron oxide that form on the surface of any metal containing iron that is exposed to air and water. It is a type of corrosion that can be highly destructive, as well as unsightly. In this article, we will share tips on how to prevent rust.The rusting process begins when iron reacts with oxygen in the presence of water, saltwater, acids, or other harsh chemicals. As the iron oxide flakes off the metal surface, it exposes fresh iron molecules, which continue the reaction process. Eventually, large areas of rust form that may cause the entire metal structure to disintegrate.
A ferrous metal is one that contains iron and only iron can rust. Common ferrous metals include carbon steel, alloy steel, and stainless steel. Non-ferrous metals, such as aluminum and copper, contain little if any iron, and so cannot rust, though they can corrode.
1. Keep It Clean and Dry
Water is enemy number one when it comes to rust, because it’s the oxygen in water molecules that combines with iron to form iron oxide. That’s why metals left outdoors, such as cars, gates, or tanks, are more likely to rust. If the object is located in a humid indoors environment, such as a garage or basement, install a dehumidifier. Any type of mud or dirt adhered to the surface can hold water, so it’s important to keep metals clean.2. Prevent Scratches
Scratches or cracks in the metal expose more metal and hold water, allowing it to remain in contact with the iron. This is why cold rolled steel is more corrosion resistant than hot rolled steel, because cold rolling creates a smoother surface without texture that can trap and hold water.
3. Apply A Protective Coating
Dipping metal objects, such as clocks, into a bluing solution of water, sodium hydroxide, and potassium nitrate, provides strong corrosion resistance. Commercially available rust prevention products in the form of aerosol sprays or cloth wipes also can protect metal objects, including tools, outdoor gear, vehicles, and large metal parts.
4. Use Stainless Steel
Stainless steel alloys contain iron, but it resists rust because it also contains a high percentage of chromium which is even more reactive than iron. The chromium in the alloy oxidizes quickly to form a protective layer of chromium oxide on the metal surface which prevents oxygen from reaching the underlying steel.
5. Use Galvanized Metal
Galvanization is a process used to preserve steel rust-free for many years. In the galvanizing process, a piece of steel is coated with liquid zinc. The zinc protects the steel in three different ways. First, the zinc coating acts as a barrier preventing oxygen and water from reaching the steel. Second, even if the coating is scratched off, the zinc continues to protect nearby areas of the metal through cathodic protection. And third, zinc is highly reactive to oxygen and quickly forms a protective coating of zinc oxide which prevents the iron from further oxidation.
6. Regular Maintenance
Because rust spreads quickly, it’s important to scrape it off as soon as it appears. Then, scrub with warm water and soap and apply a metal conditioner or other protective coating to prevent further oxidation. If necessary, apply a new coat of paint to the area.





Comments
Post a Comment